A step-by-step approach to achieve novel food approval is essential for a successful launch
A proprietary Vitamin D2 mushroom powder has recently been approved as a novel food by EFSA (European Food Safety Authority), giving food & beverage manufacturers the opportunity to add it to a wide variety of products to enhance the vitamin D2 content. Applying for novel food status isn’t straight forward and ingredient companies need to be aware of the processes to bring an ingredient to market. Companies that have the most success will be those that adopt a step-by-step approach.

Novel food
Vitamin D2 is produced in fungi and yeast by UVB-exposure of ergosterol (provitamin D2) (1). However, this rarely occurs as mushrooms are commonly cultivated in the dark (2). The recently-approved novel food vitamin D2 mushroom powder uses controlled ultraviolet (UV) irradiation to enhance the concentration of vitamin D2 in mushrooms. The new food has been approved to be consumed by the general population in a variety of foods and beverages, as well as for use in Foods for Special Medical Purposes (FSMPs) and as a food supplement for individuals above 1 year old.
Proprietary use
Because the application was authorised on the basis of proprietary data, only the applicant can place this particular vitamin D2 mushroom powder on the market within the Union during a period of five years from 24 January 2023 (3).
What are novel foods and ingredients?
Novel foods are any foods or ingredients that ‘have not been widely consumed by people in the UK or European Union (EU) before May 1997’ (4). They could be foods or ingredients that are commonly eaten outside of Europe, extracts from foods (such as botanical extracts), or food manufactured from new production technologies.
With demand for natural, sustainable plant-based products remaining strong, and the therapeutic properties of certain botanicals and plants, it’s easy to see the attraction of harnessing their benefits in food and drink applications. An increasing number of manufacturers are utilising unique processes to unleash the potential of plants, as well as providing for the next generation of consumers. However, unless a thorough step-by-step approach has been followed, this potential won’t come to fruition.
Step-by-step approach to novel food approval
Novel foods require a comprehensive understanding of their composition, history of use and toxicology, among other requirements, to be approved for use. This can be perceived as a big hurdle to innovation.
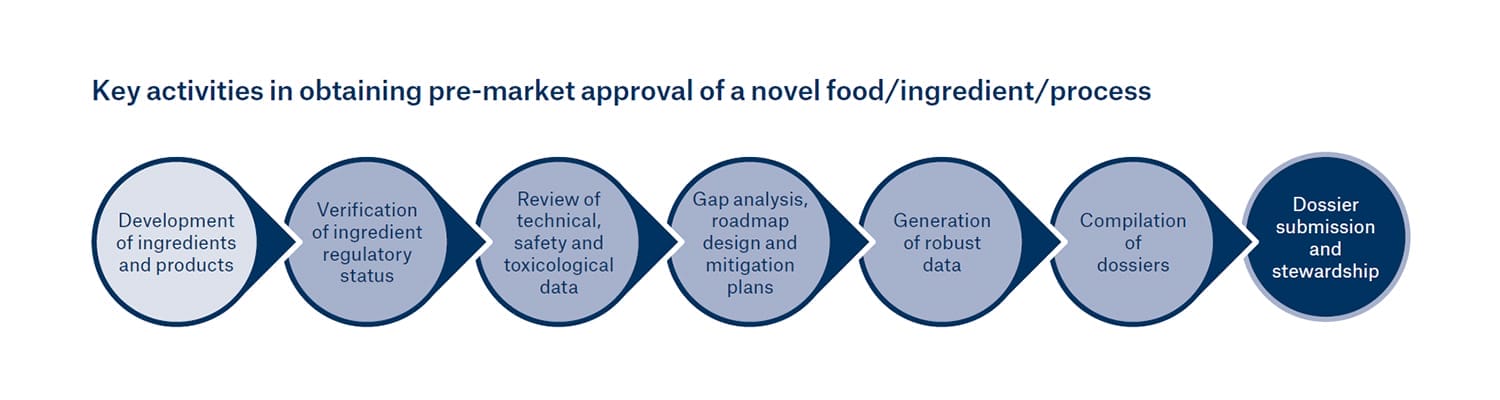
Leatherhead Food Research has identified seven key steps for companies to take in obtaining pre-market approval for novel foods/ingredients:
- Development of ingredients and products
- Verification of ingredient regulatory status
- Review of technical, safety and toxicological data
- Gap analysis, roadmap design and mitigation plans
- Generation of robust data
- Compilation of dossiers
- Dossier submission and stewardship
Support with pre-market approval
To learn more about the steps in obtaining pre-market approval of novel and new ingredients/foods/beverages, please get in touch at [email protected]. Leatherhead’s experienced team of scientists and regulatory experts, including former regulators and industry advocates, as well as toxicologists from our sister company TSG Consulting, can support you every step of the way. Follow the link to learn more about Leatherhead’s novel food dossier services.
References
(1) Jäpelt RB, Jakobsen J (2013) Vitamin D in plants: a review of occurrence, analysis, and biosynthesis. Front Plant Sci, 4:136. doi:10.3389/fpls.2013.00136
(2) Turck D et al. (2021) Safety of Vitamin D2 mushroom powder (Agaricus bisporus) as a Novel food pursuant to Regulation (EU) 2015/2283. EFSA Journal, 19:4. doi.org/10.2903/j.efsa.2021.6516
(3) COMMISSION IMPLEMENTING REGULATION (EU) 2023/4 of 3 January 2023 authorising the placing on the market of vitamin D2 mushroom powder as a novel food and amending Implementing Regulation (EU) 2017/2470 (2023) Official Journal of the European Union, L2, pp 3-8. Accessed: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32023R0004
(4) REGULATION (EU) 2015/ 2283 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/ 2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/ 97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/ 2001. Official Journal of the European Union, L327 pp1-22. Accessed: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R2283&from=EN